Fluorescence resonance energy transfer (FRET), the nonradiative transfer of excited state energy from one fluorophore to another. It is a popular tool to study equilibrium and dynamical properties of polymers and biopolymers in condensed phase. The evolution of FRET has paralleled its application in immunology. It has since been used to study receptors in cell populations by flow cytometry. Recently, with the development of digital microscopy, FRET has been applied to analysis of molecular interactions at the level of single cells, cell organelles, and single molecules. FRET microscopy is now a standard tool for investigating inter- and intramolecular distances at the nanometer scale.
Fluorescence resonance energy transfer is a distance-dependent physical process, by which energy is transferred nonradiatively from an excited molecular fluorophore (the donor) to another fluorophore (the acceptor) by means of intermolecular long-range dipole–dipole coupling. FRET can be an accurate measurement of molecular proximity at angstrom distances (10–100 Å) and highly efficient if the donor and acceptor are positioned within the Forster radius (the distance at which half the excitation energy of the donor is transferred to the acceptor, typically 3–6 nm). In addition, the emission spectrum of a donor fluorophore significantly overlaps (>30%) the absorption spectrum of an acceptor. The efficiency of FRET is dependent on the inverse sixth power of intermolecular separation.[1] It indicates the percentage of the excitation photons that contribute to FRET. ![FRET[1] FRET[1]](http://lh4.ggpht.com/_UgmFKuaBrto/TbHQn92PvzI/AAAAAAAAA-w/8fdrJvUn-8k/FRET%5B1%5D_thumb%5B19%5D.jpg?imgmax=800)
The molecular processes underlying FRET are illustrated in Figure 4. The first step involves absorption of energy by the donor molecule, resulting in excitation from the ground state, S0D, to an excited singlet state, S1D. Several excited states are available to the donor; however, vibrational relaxation to S1D by internal conversion is rapid, ensuring that a majority of emission occurs from this state. Several fates are possible for the excited donor, including spontaneous emission and nonradiative processes. If a suitable acceptor fluorophore is nearby, then nonradiative energy transfer between the donor and acceptor can occur. This transfer involves a resonance between the singlet-singlet electronic transitions of the two fluorophores, generated by coupling of the emission transition dipole moment of the donor and the absorption transition dipole moment of the acceptor. Thus, the efficiency of FRET and the range of distances over which it can be observed are determined by the spectral properties of a given donor acceptor pair.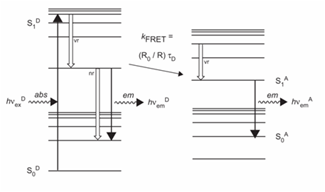
Appropriate donor and acceptor probes are selected on the basis of their absorption and emission spectral characteristics, as well as based on their selective adsorption onto specific substrates. For optimal resonance energy transfer the donor emission spectrum should substantially overlap the absorption spectrum of the acceptor. The first member of the FRET pair functions as an energy donor and the second member of the FRET pair function as an energy acceptor. When a system contains two fluorescent species such that the emission spectrum of the first (the donor) overlaps the absorption spectrum of the second (the acceptor), the energy absorbed by the donor may be transferred to the acceptor over a distance that depends on the overlap integral between the donor emission and the acceptor absorption spectra. This phenomenon may be utilized in a variety of ways to characterize the distance between the two chromophores.
[1] Stryer L., Fluorescence energy transfer as a spectroscopic ruler., Annual Review of biochemistry.,1978